Presentation of the 2016 Eppendorf Award for Young European Investigator to Adrian Liston
 Monday, June 6, 2016 at 11:15AM
Monday, June 6, 2016 at 11:15AM This year’s research prize awarded by Eppendorf goes to Belgium
 In 2016, Eppendorf AG, the Hamburg life science company is presenting its highly prestigious research prize for the 21st time. The independent Eppendorf Award Jury chaired by Prof. Reinhard Jahn selected Prof. Adrian Liston (Group leader at VIB Translational Immunology Lab, University of Leuven, Belgium) as the 2016 winner of the Eppendorf Award for Young European Investigators.
In 2016, Eppendorf AG, the Hamburg life science company is presenting its highly prestigious research prize for the 21st time. The independent Eppendorf Award Jury chaired by Prof. Reinhard Jahn selected Prof. Adrian Liston (Group leader at VIB Translational Immunology Lab, University of Leuven, Belgium) as the 2016 winner of the Eppendorf Award for Young European Investigators.
The Award ceremony took place at the EMBL Advanced Training Centre in Heidelberg, Germany, on June 2, 2016. The laudatio honoring Adrian Liston’s achievements was held by the jury chairman Prof. Reinhard Jahn.
Adrian Liston, born 1980, receives the € 20,000 prize for his seminal work in elucidating key mechanisms by which the immune system avoids attacking its own organism while remaining effective against pathogens. His experiments have paved the way for understanding key steps in controlling regulatory T-cells that are critical for balancing between autoimmunity and immunosuppression. His work opens up the way for new therapeutic approaches towards diseases resulting from a dysregulated immune homeostasis.
Adrian Liston: “My laboratory studies the genetic basis of immune disease through a multi-disciplinary approach that assesses the entire cascade of events leading to disease. We use genetic approaches to identify new mutations causing primary immunodeficiencies, cellular and biochemical immunology approaches to determine the impact of these mutations on the tolerance checkpoints, and disease modelling approaches to study the process of tissue destruction that leads to pathology. Our mission is to identify the most sensitive intervention point in the disease pathway for the development of effective therapeutics… The 2016 Eppendorf Award is a great recognition of the work done by all of the amazing people in my team. I see this prize as a validation of our philosophy to keep a broad perspective of immune diseases rather than focusing in on a single pathway or technique.”

From left to right: Axel Jahns (Eppendorf AG), Reinhard Jahn (MPI for Biophysical Chemistry), Adrian Liston (VIB/KU Leuven), Maria Leptin (EMBO), Wilhelm Plüster (Eppendorf AG), Bas Poirters (Eppendorf Nederland & Eppendorf Belgium). ©EMBL Photolab
With the Eppendorf Young Investigator Award, which was established in 1995, Eppendorf AG honors outstanding work in biomedical research and supports young scientists in Europe up to the age of 35. The Eppendorf Award is presented in partnership with the scientific journal Nature. The Award winner is selected by an independent committee composed of Prof. Reinhard Jahn (Max Planck Institute for Biophysical Chemistry, Göttingen, Germany), Prof. Dieter Häussinger (Clinic for Gastroenterology, Hepatology and Infectiology, Düsseldorf, Germany), Prof. Maria Leptin (EMBO, Heidelberg, Germany), and Prof. Martin J. Lohse (Max Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany).
More information about entry details, judging procedures, and past winners can be found at www.eppendorf.com/award
 Liston lab
Liston lab Santa Cruz Biotechnology punished for violating animal ethics
 Monday, May 23, 2016 at 9:29PM
Monday, May 23, 2016 at 9:29PM Animal researchers are under intense scrutiny to make sure they abide by strict ethical guidelines. We constantly need to be trained and licensed and to justify the use of each and every animal. The "3Rs" (Replacement, Reduction and Refinement) are drilled into us from the start, and animals in scientific research have far more legal protections and oversights than animals on farms or household pets. I strongly support this regulation* - I consider myself an animal rights activist as well as an animal researcher.
This is why I am so glad to see the US government crack-down on Santa Cruz Biotechnology. Santa Cruz (a major antibody producer) illegally kept hundreds of animals in a shadow animal facility it repeatedly lied about to the inspection authorities. It subsequently racked up 31 animal rights violations and then eliminated its entire animal stock (more than 5000 animals) in an attempt to circumvent inspections. This type of (rare) bad behaviour provides ammunition against good animal research facilities, which is why I am glad to see they are essentially being shut-down with a $3.5-million fine and (more importantly) it has permanently lost its licence to sell, buy, trade or import animals. Santa Cruz is now out of the animal research community, and we are better for it.
*small proviso, I support regulation that is intended to protect animals. I do not support regulation where the intent is to provide a back-door ban on animal research by making pointless regulations that are so difficult and expensive to abide by that they essentially consistent a de facto ban
 ethics,
ethics,  scientific method
scientific method New insights into Multiple Sclerosis treatments
 Wednesday, May 11, 2016 at 3:40PM
Wednesday, May 11, 2016 at 3:40PM Multiple Sclerosis is the most common neurodegenerative disease of young adults, affecting 2.3 million people. MS is insidious. It can lie dormant for years, controlled well by treatment, but there is no cure and patients always live with the threat of another attack that takes away more of their function. One of the frustrating aspects of MS is that we have treatments, but we don't really understand them. There are plenty of drugs that work to control MS, but it is impossible to predict which drug will work well for which patient, or how long that drug will work. We don't even really understand the way that the different drugs function - essentially, it is a guessing game to find which treatment will work best in which patient; a guessing game that dangerously chews up time as the disease progresses.
In a major new study just released, the Translational Immunology laboratory teamed up with the Neuroimmunology laboratory (led by Prof An Goris) and performed the first large-scale in-depth immunological analysis of multiple MS treatments. We profiled the immune systems of 245 individuals, including untreated MS patients and MS patients being treated with four standard treatments - interferon-beta, glatiramer acetate, natalizumab, or fingolimod. Since all the treatments are effective in at least some patients, we had expected to find that each treatment ould have a similar impact on the immune system. Instead, the results were surprising - each of the treatments did something different to the immune system.
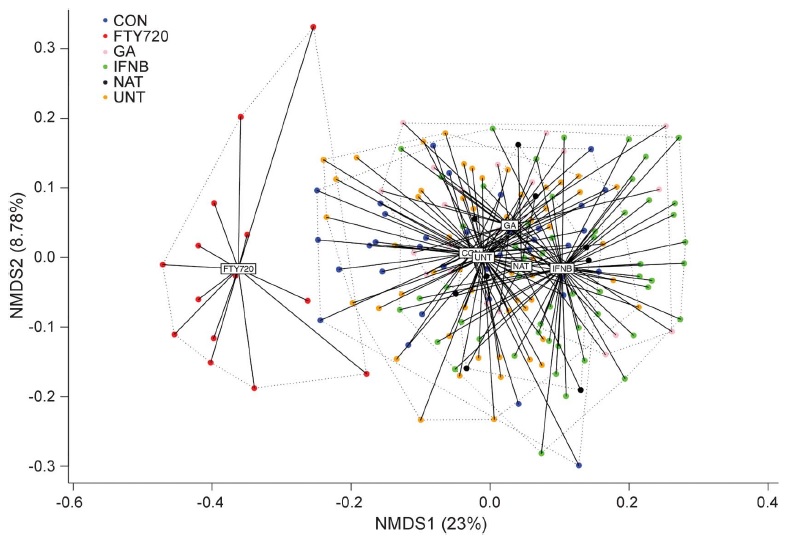
In fact, the only common response we found to MS treatment was an increase in the serum cytokine BAFF. The confusing part is that BAFF was thought to be detrimental during MS - several mouse trials found that increased BAFF drives more severe disease, while inhibiting BAFF cured disease. These mouse results were strong enough that two clinical trials had started injecting anti-BAFF antibodies into MS patients in the hope of stopping disease progress. And yet, we found that BAFF was going up in patients that were given multiple different effective MS treatments! Our model suggests that increased BAFF may actually be a protective part of MS treatment, so is it wise to give MS patients anti-BAFF? Unfortunately, our model appears to be correct, as the two trials of BAFF in MS have now been prematurally stopped, due to excessive adverse events.
There are three major lessons to be learned from our study:
First, we should look at testing drugs that increase BAFF rather than decreasing BAFF. This may be a promising avenue for treating MS in patients that do not respond to existing drugs.
Second, we should stop assuming that we understand how existing drugs work. Every drug that we give has multiple impacts on the body, and we should not assume that we know which of these impacts are the protective ones. By identifying which particular impacts are shared across multiple effective drugs, then we are more likely to be looking at the protective effects. If our study had been performed earlier, then I doubt anyone would have gone ahead and given anti-BAFF antibodies to MS patients, and these adverse events could have been avoided.
Third, further large-scale immune analyses such as ours may allow us to predict which patients will respond to which drugs best. In MS this is critical - time spent on an ineffective drug means function is lost that will not be regained - patients need the right drug as soon as possible.
You can read more about our study at Neurology: Neuroimmunology & Neuroinflammation:
Dooley*, Pauwels*, Franckaert, Smets, Garcia-Perez, Hilven, Danso-Abeam, Terbeek, Nguyen, De Muynck, Decallonne, Dubois, Liston* and Goris*. 'Immunologic profiles of multiple sclerosis treatments reveal shared early B cell alterations'. 2016 vol. 3 no. 4 e240
 Liston lab,
Liston lab,  Medicine,
Medicine,  immunology,
immunology,  neuroscience
neuroscience Flaws in scientific studies and science reporting
 Tuesday, May 10, 2016 at 8:55AM
Tuesday, May 10, 2016 at 8:55AM Activating inflammation
 Wednesday, May 4, 2016 at 9:21AM
Wednesday, May 4, 2016 at 9:21AM My recent seminar at the Cold Spring Harbor Laboratory:
 Liston lab,
Liston lab,  immunology
immunology Intolerable secretion and diabetes in tolerant transgenic mice, revisited
 Wednesday, April 27, 2016 at 6:22PM
Wednesday, April 27, 2016 at 6:22PM A new mouse model linking diabetes, insulin secretion and autoimmunity with a high-fat diet supports a shared mechanism for type 1 (T1D) and type 2 (T2D) diabetes. In this model, the protein secretion system of insulin-producing pancreatic beta cells is stressed, leading to increased beta cell apoptosis and diabetes via reduced levels of the transcription factor GLIS3, a pathogenic pathway that can be mimicked by a high-fat diet.
Read more of this commentary in Nature Genetics
 diabetes
diabetes Flemish scientists find cure for rare immune disease
 Thursday, April 21, 2016 at 8:46AM
Thursday, April 21, 2016 at 8:46AM A long wait
The disease – known as PAAND – causes severe skin lesions, muscle pain and general exhaustion. It first came to light about 10 years ago, when paediatric rheumatologist Carine Wouters (pictured right) was confronted with the case of a 13-year-old boy who was brought to the emergency department at Leuven University Hospital.
“Because of a cardiac muscle infection, the boy suffered from heart problems, but we quickly realised that the condition was part of a broader auto-inflammatory disease,” says Wouters.
To her amazement, the doctor soon discovered that other members of the young patient’s family were exhibiting similar symptoms. “But the cause remained unknown,” she says.
Unbearable pain
The boy’s father, who prefers to remain anonymous, has suffered from the same mysterious disease for most of his life. “Since I was five, I have had severe muscle pains, and I’ve suffered from extreme exhaustion,” he says. “Sometimes, I couldn’t even stand up and had to stay home from school for days, just to recover.”
He eventually finished school and found a job, but the condition made his career as a truck driver no less difficult. “The older I got, the more frequently I had to pull over to the side of the road and rest for a few hours,” he says.
The pain was so unbearable, he often couldn’t sleep through the night. As more health problems accumulated, the now 50-year-old man was forced to quit his job.
Sometimes, I couldn’t even stand up and had to stay from school for days, just to recover
- PAAND PATIENT
Altogether, 12 members from three generations of the West Flemish family are afflicted with the disease. Until now, doctors could only ease their suffering with anti-inflammatory drugs and painkillers.
Genetic mutation
The breakthrough discovery of PAAND is the result of an extensive DNA comparison between the patients’ blood and that of their family members who are not affected by the disease.
The study was led by KU Leuven professor Adrian Liston, who also works at Flanders’ life sciences research institute VIB. Liston’s team collaborated with scientists from the Walter and Eliza Hall Institute in Melbourne, Australia.
The researchers traced the cause to a mutation of a gene known as MEFV. They determined that the mutation tricks the body into responding to a bacterial skin infection, even if there isn’t one. The response causes the skin to produce an inflammatory protein called interleukin-1 beta, which causes skin lesions, fevers and pain.
“If you have the flu, the fever and exhaustion you experience are the result of your immune system putting a lot of energy into battling the infection and not having enough energy left to allow you to function normally,” Liston explains.
With PAAND, he continues, “the immune system diverts much of that energy into fighting an infection that isn’t actually there, with disastrous consequences”.
Only one of the parents needs to carry the mutation for the disease to affect their children, though it isn’t necessarily passed on to every child.
The cure
“These insights were made possible because DNA analysis has become more innovative and affordable,” explains Liston. “We also couldn’t have done it without the collaboration with our colleagues abroad.”
Once they understood how the disease operates, the scientists started looking for a treatment. They found it in a drug called anakinra, also known under the brand name Kineret. While the drug is used in treating rheumatoid arthritis, Liston found it also has the ability to block the protein that causes PAAND.
We hope that the discovery will improve the quality of life for many other people in Flanders and abroad
- DR CARINE WOUTERS
The medication is now being tested on five members of the family from West Flanders, including the father. “I hope the drug can make our lives more normal,” he says.
For the time being, his son is recovering from a second heart transplant and cannot take the medication. The scientists are planning to involve him and other patients at a later stage.
Dr Wouters, who has stood by the Flemish family over the past 10 years, is happy that their resilience has finally been rewarded. “We hope that the discovery will improve the quality of life for many other people in Flanders and abroad,” she says. Doctors in France and Lebanon have already indicated that they also have patients with PAAND.
The next steps
The treatment was first carried out in the UK because the country’s health-care system reimburses the drug anakinra. For the tests on Flemish patients, KU Leuven researchers negotiated directly with the drug’s manufacturer, the Swedish company Orphan Biovitrum.
We know about 6,000 rare diseases, and more innovative techniques could help us to identify new ones
- DR CARINE WOUTERS
The two scientists have called on Belgium’s federal health minister Maggie De Block to focus more attention on diseases like PAAND. “Rare diseases are not rare,” Wouters says. “We know some 6,000 of them, and more innovative techniques could help us to identify new ones.”
According to Liston, more newborn babies should also be screened for immune system deficiencies. This, he says, could help prevent medical conditions later in life, which “not only affect the person’s overall health, but result in high costs to the social security system”.
 Liston lab,
Liston lab,  Medicine,
Medicine,  immunology
immunology Coauthors
 Monday, April 18, 2016 at 12:59PM
Monday, April 18, 2016 at 12:59PM 
All my coauthors from 110 publications. You can see the productivity of lab members, and also our most frequent collaborators - Michelle Linterman, Daniel Gray, Sylvie Lesage, Chris Goodnow, Carine Wouters
 Liston lab
Liston lab BioCentury Innovations
 Wednesday, April 13, 2016 at 1:38PM
Wednesday, April 13, 2016 at 1:38PM Our work was highlighted by BioCentury Innovations as a novel strategy to screen for anti-diabetic drugs:

 Liston lab,
Liston lab,  diabetes
diabetes 






