A New Hope for IPEX patients
 Tuesday, May 9, 2023 at 10:51PM
Tuesday, May 9, 2023 at 10:51PM A new paper from our lab suggests a novel approach to treating IPEX patients. IPEX is a rare severe primary immunodeficiency, caused by a genetic deficiency in the gene FOXP3, which results in a lack of anti-inflammatory regulatory T cells.
IPEX is usually fatal in childhood if left untreated. The only cure is a haematopoietic stem cell transplantation, however patients are often so sick from autoimmunity that they are in poor condition to receive a transplant. The patients are put on symptomatic support (hormonal and nutritional supplements to compensate for the damaged organs) and immunosuppressive drugs to reduce further damage. These immunosuppressive drugs are typically combinations of cyclosporine A, tacrolimus, rapamycin and corticosteroids, although recently biologics such as orthoclone have been suggested. Unfortunately the patient cohort has been too small and heterogeneous to allow a proper clinical trials as to which immunosuppression regimen works best.
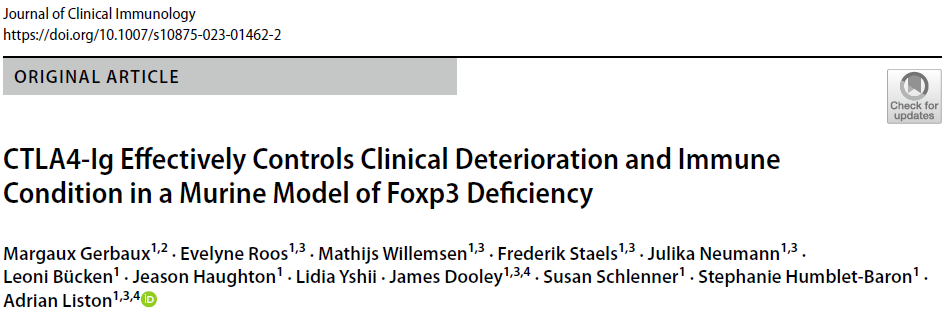
We sought to answer this by turning to the mouse model - also with a genetic deficiency in Foxp3 and a lack of regulatory T cells. We developed a comprehensive pathology scoring system for the model that takes into account the multiple different autoimmune symptoms, and then tested in a side-by-side comparison rapamycin (the most common standard treatment), anti-CD4 antibody (analgous to orthoclone in its proposed approach) and CTLA4-Ig (based on our prior work on CTLA4-Ig compensating well for Treg-deficiency).
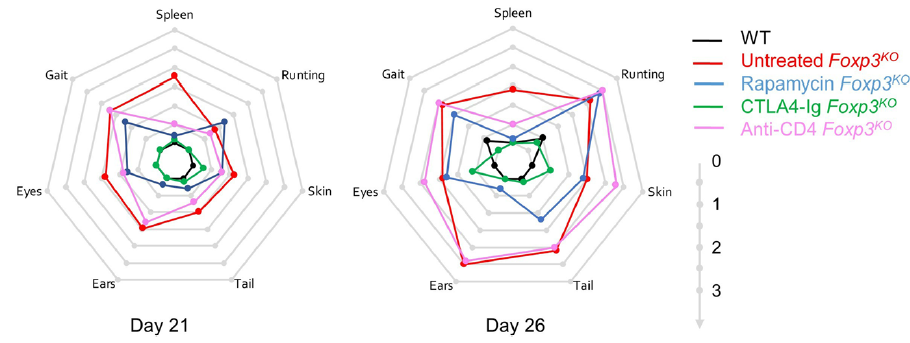
The results were striking. As seen in patients, rapamcyin cleared up some of the skin pathology, but otherwise it had little impact on the course of pathology in the mice. Anti-CD4 antibody prevented many of the immunology symptoms, but again, didn't actually improve the aggregate health outcomes of the mice. CTLA4-Ig, by contrast, improved essentially every parameter - the mice started gaining weight like normal, improved their serology, skin pathology and organ histology - and had greatly improved life-spans. Most importantly, the overall condition of the CTLA4-Ig-treated mice improved to such an extent that they were capable of supporting curative bone-marrow transplants: survival improved from 50% to 100% in mice given CTLA4-Ig prior to transplantation.
There are caveats to every disease model, however we believe this is sufficient evidence to strongly consider a clinical trial of CTLA4-Ig (abatacept) in IPEX patients. The genetic and cellular defects are entirely conserved between mouse and human in this case, and the drug is in widespread use in patients for other autoimmune conditions (such as arthritis). We know that there are IPEX patients who respond poorly to the current standard treatments and need to improve their condition before receiving a bone-marrow transplant. CTLA4-Ig treatment could be the bridge that these patients need to the curative transplantation!
Thanks to the Jeffrey Modell Foundation for sponsoring this study, which was done in collaboration with lab alumni Prof Stephanie Humblet-Baron at the University of Leuven in Belgium. Check out the full paper at the Journal of Clinical Immunology!
 Liston lab,
Liston lab,  Medicine,
Medicine,  immunology
immunology 




Reader Comments (2)
there are IPEX patients who respond poorly to the current standard treatments and need to improve their condition
Really excellent and profitable article! I appreciate you giving these to me and to everyone else.