Go with the flow – a new algorithm streamlines and improves flow cytometry analysis
 Monday, May 17, 2021 at 12:10PM
Monday, May 17, 2021 at 12:10PM  Key points:
Key points:
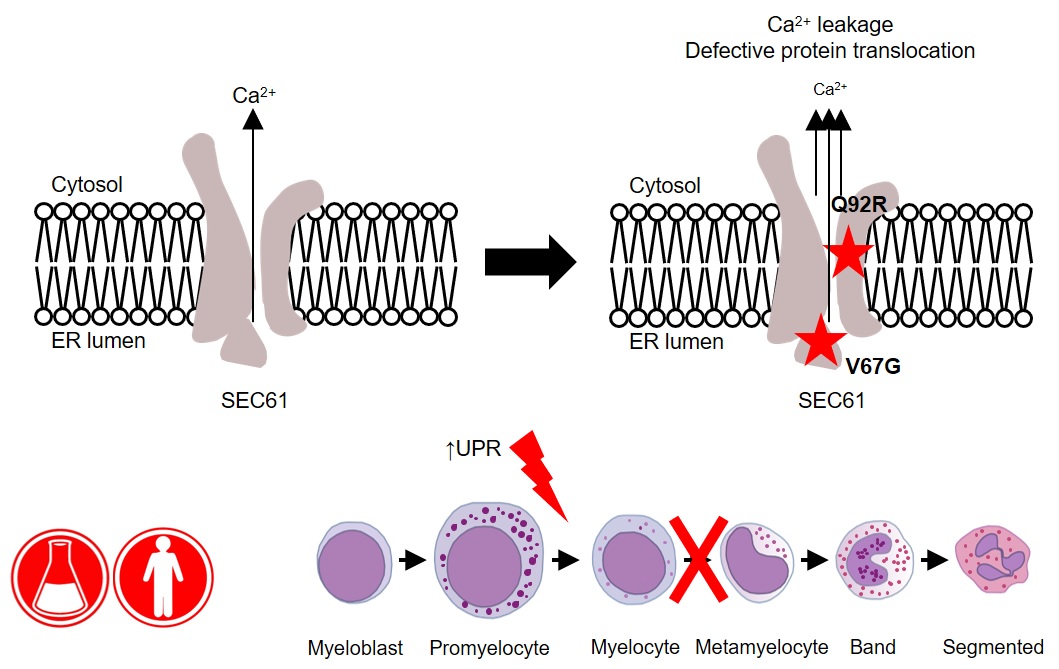
- A new algorithm developed by researchers at the Babraham Institute provides a fast and effective way to reduce errors in flow cytometry data analysis, overcoming a major restriction on harnessing the full potential of the power of flow cytometry in cell analysis.
- The tool, called AutoSpill, addresses the problem of overlapping signals and autofluoresence, which can be misinterpreted as genuine results.
- Researchers can use the tool, available online and through the software package FlowJo, to easily reduce compensation errors in their flow cytometry data.
Flow cytometry is a key investigative tool used in biomedical research, allowing researchers to identify, separate and study cells according to their characteristics, often working with cell samples containing millions of cells at an analysis pace of a million cells per minute. Cell identification is achieved by labelling cells with fluorescent tags. As with personal gadgets and devices, innovation in molecular biology technologies isn’t standing still. Advances in flow cytometry have allowed scientists to gather data on a growing number of parameters, simultaneously detecting over 30 different tags at a time to allow more sophisticated analyses and much deeper levels of insight. However, while flow cytometry equipment has been updated, the accompanying computational requirements have received less attention, until now. AutoSpill, an algorithm developed by researchers at the Babraham Institute and the VIB Center for Brain Research, brings data processing in line with state-of-the-art machines, simplifying data analysis and increasing accuracy. The new technique is published in Nature Communications today.
Immunology programme senior group leader Prof. Adrian Liston, explained: "Flow cytometry is a foundational technology across many different biomedical research areas, and is a key diagnostic tool in immunology, haematology and oncology. Despite the technical progress over the past decades, the technology has been held back by the mathematical processing of the data. Our new approach reduces error by 100,000-fold, making research and diagnostics more accurate. The collaboration with FlowJo has enabled us to instantly reach 80,000 users. It is very gratifying to see computational biology have a direct and real impact on research and diagnostics."
Using multiple fluorescent signals raises a key issue in flow cytometry called spillover. Spillover occurs because each tag, called a fluorophore, emits light within a range of wavelengths, giving it a unique colour. When multiple fluorophores are used, the signals begin to overlap. To accurately distinguish between two distinct fluorophore signals, researchers must process their data to compensate. Because flow cytometry uses so many different colour tags on each cell, the spillover between colours quickly accumulates, limiting scientists’ power to draw reliable conclusions from their results. The processing of data to remove the spillover between the different colours, known as compensation, is necessary for all flow cytometry experiments. Current methods require many hours of manual work, but AutoSpill reduces the process to minutes.
Dr Rachael Walker, Head of the Institute Flow Cytometry facility, commented: “The new AutoSpill Fluorescence Compensation algorithm is a great tool for quick, simple and accurate compensation. It allows compensation to be accurately calculated on samples where the traditional algorithm is difficult to use. AutoSpill’s integration into the FlowJo post-acquisition software highlights the importance of this new compensation method.”
Another limitation of flow cytometry is autofluoresence, fluorescence produced naturally by cells. The removal of these artefacts by AutoSpill is particularly useful for cancer biologists as tumour cells are high in autofluorescence, which can confuse identification of the type of tumour cell present. By solving these sources of error, AutoSpill can help remove false positives from cell analyses, ensuring more accurate data interpretations.
AutoSpill is available through open source code and a freely-available web service. AutoSpill, and a complementary related tool, AutoSpread, are also available in FlowJo v.10.7. Dr John Quinn, Director of Science and Product Development, FlowJo added: “AutoSpill & AutoSpread have been a revelation for FlowJo users. Compensation has long been one of the most perplexing aspects of cytometry, with the most critical requirement being pristine compensation controls collected for each and every parameter in an experiment. Overall, the combination of these two tools makes compensation both easier and more robust. As an indicator of the popularity of this new approach, the webinar held in conjunction with Nature to introduce AutoSpill / AutoSpread in FlowJo has been viewed over 400 times after the initial live event. We at FlowJo believe the AutoSpill / AutoSpread approach will be the primary means of approaching compensation moving forward.”