I was interested by Ruairi Mackenzie from Technology Networks for World Multiple Sclerosis Day. Here is the resulting article:
World Multiple Sclerosis (MS) Day recognizes the millions of people worldwide who are affected by this neuroimmunological disease. The campaign site for World MS Day 2022 strikes an optimistic chord, seeing the date as a chance to “celebrate global solidarity and hope for the future”. This year, there is more reason to buy into that optimism that ever before.
Recognizing the immune basis of MS
Adrian Liston, a group leader at the Babraham Institute, based near Cambridge, UK, is well-placed to explain that sunny outlook. He first studied MS as part of an undergraduate project. Over the two decades since, Liston has continued to research in the MS field, watching science’s understanding of the disease deepen.
“I think the most profound change has been the recognition that MS is an immune-mediated disease,” says Liston. The symptoms of MS are neurological – patients experience a range of sensory and motor conditions, including fatigue, numbness, spasms and weakness and loss of control over muscle movement or function. While MS has long been thought to include an immune component – Jean Martin Charcot, the French doctor who first described the disease, noted the presence of immune cells in patients’ spinal cords – it was only recently that the immune-mediation of the disease was fully understood. It’s now recognized that the neurological symptoms are a consequence of immune cells infiltrating the brain and attacking the myelin sheath coating that permits normal nerve cell conduction.
Part of that understanding came from the galloping pace of genetics research. The neurological symptoms of MS are in contrast with its genetic signature, explains Liston. From a geneticist’s point of view, “MS looks a lot like Type 1 diabetes, or any of these other autoimmune diseases, and the same genes are controlling it,” he says.
These genetic insights were added to a rapidly growing body of preclinical research. Experimental autoimmune encephalomyelitis (EAE) is an inflammatory autoimmune disease seen in animals that mimics the disease course of MS. This model proved essential for the development of drugs for MS. Liston explains that these drugs came in two waves. The first was heralded by the approval of interferon beta-1b in 1993, the first drug capable of altering the course of MS. The interferons, which reduce the number of immune cells crossing the blood-brain barrier, showed success at improving MS symptoms. This provided direct evidence that adopting an immune-targeting approach could help patients.
A new generation of drugs
For some, these early drugs have proved enduringly beneficial and remain their main course of treatment years later. MS drug development, however, continued to refine the targeting of the immune system. “What we really we have now is the second wave of drug generation, where we have much more sophisticated immune-modulating molecules, and we can really target very specific pathways of cells that are causing the damage,” says Liston. These new drugs (there are 23 FDA-approved mediations for MS as of 2022) work better and for longer.
Liston says that an MS patient today can expect fewer symptoms and far longer periods of good health than in the past. 85% of MS patients have a relapsing-remitting form of the disease, which sees symptoms wax and wane. While 20 years ago MS may have induced multiple relapses each year, with potentially permanent loss of function a risk each time, today a patient that responds well to the latest treatments can expect to go five or even ten years without any further relapses, says Liston: “It gives them a life well beyond diagnosis, which wasn't the case 20 years ago.”
The focus of these treatments is to minimize the damage of any immune attack on the brain. Can we also restore function lost during these attacks? Research in this area is progressing, if at a much slower rate. Data presented in 2020 showed that a compound, bexarotene (full disclosure: I’ve published research on this drug myself) was shown to restore some of the myelin lost during the disease course. Side effects of the drug and limited clinical impact meant bexarotene was not taken forward to approval. The challenges of restoring the damaged brain are significant, but this research shows that, in principle, healing the brain’s myelin might one day be possible.
A vaccine to prevent MS?
In the meantime, other findings have pointed a way to intercept MS earlier, stopping the disease in its tracks before it can ever cause damage to the brain.
It all begins with a virus.
Epstein-Barr virus (EBV), a type of herpesvirus, has long been associated with MS – studies had noted a higher risk of contracting MS after previously having infectious mononucleosis (IM), a disease caused by EBV – but a “smoking gun” had been hard to identify.
This is because up to 95% of the adult population have EBV, which is an incredibly successful virus that, after infecting a host, can lay dormant for years. Designing the right sort of study to test whether getting EBV would later increase your MS risk was therefore a huge logistical challenge. In January, however, researchers at Harvard Medical School met that challenge. Using a longitudinal method, the team collected blood serum samples from US military personnel, who are required to submit a blood serum sample at the start of and then after every two years of their service.
With samples from 10 million different people stored, the study was easily able to identify individuals who didn’t have EBV during their first sample, as well as those who developed MS during the course of their service. The study showed that, of 35 individuals who tested negative for EBV on enrolling, all but one of them went on to become infected with EBV before developing MS.
This corresponds to a 32-fold increased risk of developing MS. To put this in perspective, the strongest genetic risk factor for MS – which involves having a set of particular immune genes – confers a three-fold risk. This association is so strong, that the study, in Liston’s opinion, “finished the argument” on whether EBV plays a causal role in MS. The underlying theory is that, in some individuals, the body responds to the presence of the virus by mistakenly attacking the brain’s myelin sheath, triggering MS’s symptoms.
“Incredibly profound” implications
The finding and its implications, says Liston, are “incredibly profound”: “I'd say the best analogy is of human papilloma virus (HPV) and cervical cancer. Cervical cancer and some anal cancers as well are largely caused by infections with the virus, HPV. Now that knowledge was very controversial for a long time.”
The evidence linking HPV and cervical cancer is now solid, Liston says: “Most people who have the virus never get the cancer. But what that allows us to do is then go and develop vaccines for HPV. By preventing the infection, you essentially prevent the cancer formation.”
If a similar approach could be taken with EBV – vaccinating every child against the virus before they are infected – future generations could essentially be protected from ever acquiring MS. That’s a hugely exciting prospect. There is much more research to be done, however, before that reality is met. Liston points out that EBV “is not a trivial virus to attack” – it tends to hide within patients’ B cells and is happy to remain concealed there for a patient’s lifetime. Much more work will need to be conducted on how to target EBV and on the steps between infection and symptoms of MS.
The future of MS research
For now, says Liston, anyone interested in following the progress of the MS field should pay special attention to studies that improve the personalization of existing treatments. “We really want to be able to match up which medication is going to work on which person. In the case of MS, that is probably the single most important thing,” he explains. Currently, patients can endure multiple relapses while testing out which treatment works best for them. Work into matching patients with certain backgrounds and disease progressions with an appropriate drug could be hugely significant, says Liston.
The other exciting advance he mentions is the creation of better targeted drugs. Despite the advances of second-generation MS drugs, Liston says that in terms of specificity, they are essentially immune sledgehammers.
“In someone with MS, only 0.1% of their white blood cells are dangerous,” he says. But current treatments hit that 0.1% alongside a vast swathe of the rest of the immune system, which can have extreme side effects. New generations of drugs, Liston explains, will be better targeted at immune culprits. One field, antigen-specific tolerance work, looks to suppress the incorrect response by confused immune cells, restoring the balance of the immune system without impairing it from its important role of protecting our body against outside threats.
Time to be excited about the present
Is Liston hopeful about the future of MS research? “It’s not even just the future,” he responds, “I’m actually quite positive about the present of MS compared to where we were 20 years ago.”
The promise that drugs targeting the immune system could help MS patients has been well met. “We're not talking about cold fusion, where they've been promising boundless energy in 10 years’ time, and promising that for 40 years, we’re talking about a place where the promises have been delivered over and over,” says Liston. He believes that patients are going to continue to have longer, healthier lives as drugs improve and become smarter and more targeted.
The only note of caution Liston sounds is in managing our expectations: “We're not going to have these type of ‘Eureka!’ moments where there's one tablet that just cures MS, but we will be coming closer and closer to a place where there will be a treatment regime that works on almost every patient and is almost perfect.”
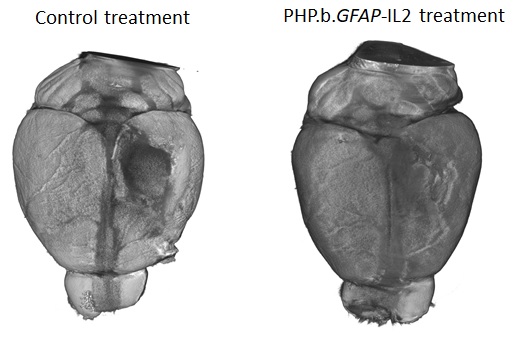
Those advances will partly come from innovative use of the latest techniques – Liston’s lab recently published work where gene therapy was used to edit a small fraction of cells in the brain. The edit caused these cells to produce a pro-survival molecule in the surrounding brain area. This is turn helped increase the numbers of regulatory T cell (Tregs) – a cell able to suppress damaging immune responses – in the brain. In animal models, the longer-living Tregs were able to not only improve MS-like symptoms but facilitate quicker healing from brain injury.
Neurological disease can so often seem intractable, beset by the complications of the brain and our still juvenile understanding of how the biological wonders in our heads function and fail. But on World MS Day 2022, there is plenty good news, at least in one small corner of brain medicine. “This is a very hopeful time for patients,” Liston concludes.
 Tuesday, June 14, 2022 at 5:20PM
Tuesday, June 14, 2022 at 5:20PM  Liston lab,
Liston lab,  immunology
immunology