Battle Robots of the Blood reading
 Thursday, February 4, 2021 at 1:48PM
Thursday, February 4, 2021 at 1:48PM Me and Hayden read "Battle Robots of the Blood" together.
Virus Fighter
Build a virus or fight a pandemic!
Maya's Marvellous Medicine
Battle Robots of the Blood

Just for Kids! All about Coronavirus
 Thursday, February 4, 2021 at 1:48PM
Thursday, February 4, 2021 at 1:48PM Me and Hayden read "Battle Robots of the Blood" together.
 Monday, January 25, 2021 at 3:08PM
Monday, January 25, 2021 at 3:08PM The project wishes to diagnose rare autoinflammatory systemic diseases through the identification of biomarkers
In December 2020 a new project has been launched in the University Hospitals Leuven. The ImmunAID project aims to identify new tools for the diagnosis of systemic auto-inflammatory diseases (SAID). SAID are a complex and evolving group of rare diseases characterised by extensive clinical and biological inflammation. These conditions are caused by a dysregulation of the innate immune system leading to a release of immune cells and mediators provoking fevers, tissue and organ inflammation and damage.
Sometimes it is difficult for the physicians to make a correct diagnosis, since the main symptoms of these diseases (such as fever, rash, joint pain, etc.) are also present in many other conditions. Thus, a patient may have received on average up to 5 inappropriate or ineffective treatments before being properly diagnosed, having a great impact on their health and quality of life. The aim of ImmunAid is to understand the mechanisms that drive the pathology in order to provide better diagnosis and care for patients with these rare but potentially devastating diseases.
An unprecedented body of clinical and biological data in the field of SAID
This new project aims to find new and more effective ways to diagnose SAID. While it is already known that some SAID are due to specific genetic mutations, a large number of SAID can only be detected by a set of clinical signs and symptoms and after other diagnostic possibilities have been excluded. Since SAID are rare conditions, a large group of patients suffering from various SAID is being recruited throughout Europe. As such, the ImmunAID cohort represents a very important tool for researchers defining biological fingerprints, or biomarkers, specific to distinct SAID.
The team expects to find a set of biological features common to all SAID, which will allow to quickly confirm or refute the diagnosis of suspected autoinflammatory syndrome. In addition, for each SAID, a list of characteristic biomarkers and an algorithm will be generated to allow the physician to make an appropriate diagnostic assessment.
In order to achieve the project's objectives, biological samples collected from the patients will be analysed in a European-wide research network by set of state-of-the-art technologies and will generate an unprecedented amount of data (genomics, transcriptomics, proteomics and microbiome). Simultaneously, other analyses will focus on immune cells, molecular mechanisms and specific agents of the immune system (cytokines, etc.). All data generated will be subjected to artificial intelligence and modelling analysis.
Prof. Carine Wouters, paediatric rheumatologist at the University Hospitals Leuven, is highly committed to the success of the project "We are delighted and proud to be able to work with ImmunAID partners as it represents a unique opportunity for the European scientific community to advance research in an important field of rare diseases that can only be tackled at large scale. We will do our best to come up with meaningful results that will improve patients’ diagnosis and medical care.”
Leuven teams are the forefront of the project
The teams of the Leuven University Projects are at the forefront of the project. The activities carried out in the Belgian centre will be two-fold. First, the team from professor Carine Wouters and professor Steven Vanderschueren will be in charge of recruiting patients suffering from monogenic SAID (FMF, CAPS, TRAPS, MKD) or genetically-undiagnosed SAID (Still disease, neutrophilic dermatosis, Schnitzler syndrome, Takayasu arteritis, Kawasaki disease, Behçet disease, chronic osteitis, recurrent pericarditis and chronic systemic inflammation of unknown origin).
Second, professor Wouters, professor Patrick Matthys and professor Paul Proost from the Rega Institute and KU Leuven department for Microbiology, Immunology and Transplantation will be involved in the biochemical and biological analysis of the samples. The team of Carine Wouters and Patrick Matthys will apply their extensive knowledge on Natural Killer cells to identify and characterize their possible altered activity in SAID patients. On the other hand, the team of Paul Proost will study whether modifications of messengers of the immune system (cytokines and chemokines) in patients play a role in regulation of the inflammation processes. The team of professor Stephanie Humblet-Baron and professor Adrian Liston will analyse in-depth the immune cellular compartment of the blood of affected patients in addition to genetic investigation in order to identify new genes responsible for SAID.
These activities are intended to gain insight into the mechanisms triggering the aberrant behaviour of the autoinflammation process. The results will be pooled with other analyses from other European research laboratories to help identify biomarkers of the diseases and possible therapeutic interventions.
Regarding the ImmunAID project: ImmunAID is a research project (www.immunaid.eu), which aims to identify a set of disease-specific biomarkers to confirm the diagnosis of SAID. ImmunAID is implemented by a large consortium (25 partners in 12 European countries) and has been funded with € 15.8 million by the European Commission. The ImmunAID project has received funding from the European Union's Horizon 2020 research and innovation programme under Grant Agreement No. 779295.
 Liston lab,
Liston lab,  Medicine,
Medicine,  immunology
immunology  Wednesday, January 13, 2021 at 10:54AM
Wednesday, January 13, 2021 at 10:54AM If anyone is interested in our lab's work on IL-2 cytokine networks, I just gave a seminar on the topic, which I am putting up here:
It is a new talk for me, and was an interesting one to write. I started to work on IL-2 right at the start of my PhD. I was very keen to return to the topic when I opened my own lab in Belgium (2009), with one of my first PhD students (Dr Wim Pierson) working on the niche-sensing and niche-filling negative feedback loop that provides a stable number of Tregs in the system. (An excellent collaboration with one of my favourite immunologists, Prof Daniel Gray from WEHI, Australia).
Then Prof Stephanie Humblet-Baron joined my lab for a post-doc, wanting to work on a disease known as Familial hemophagocytic lymphohistiocytosis (FHL). At the time, this was thought to be a disease of CD8 hyper-activation and IFN-gamma. Thanks to great work by Stephanie, in mouse and human, we now know that FHL is only partly driven by IFN-gamma, and instead a key part of pathogenesis comes from flipping the negative feedback loop between IL-2 and Tregs into a postivie feedback loop between IL-2 and CD8 T cells.
Right back in 2009 we started to work on a new genetic switch that would let us turn IL-2 on in different cell types. At first I just wanted to see what would happen if Tregs could make their own IL-2. By breaking that dependency on exogenous IL-2 do you get a run-away Treg reaction? (answer: yes, yes you do). Once we finally made the mice, however, it just opened so many different doors. What happens if CD8 T cells make their own IL-2? How about NK cells, dendritic cells, B cells? What if we turn it on in different organs? It has really been a phenomenal mouse that just kept on delivering interesting results. Dr James Dooley led a team working on the mouse, and more recently Dr Carly Whyte drove the project to publication. Or, at least, pre-publication - you can see the paper here on BioRxiv. So many interesting aspects of IL-2 biology were illuminated by this work - easiest to show in a circuit diagram:

I hope you enjoy the seminar. Keep an ear out for the muffled bang at the 29 minute mark. It doesn't sound like much on the audio feed, but across Cambridge we all jumped up as the windows rattled and the building shuddered. I fumbled the graph on this slide, calling Tregs Tconv by mistake, wondering if an explosion had gone off downstairs. Fortunately it was just a sonic boom as fighter jets scrambled overhead.
 Liston lab,
Liston lab,  immunology
immunology  Tuesday, January 12, 2021 at 8:55PM
Tuesday, January 12, 2021 at 8:55PM 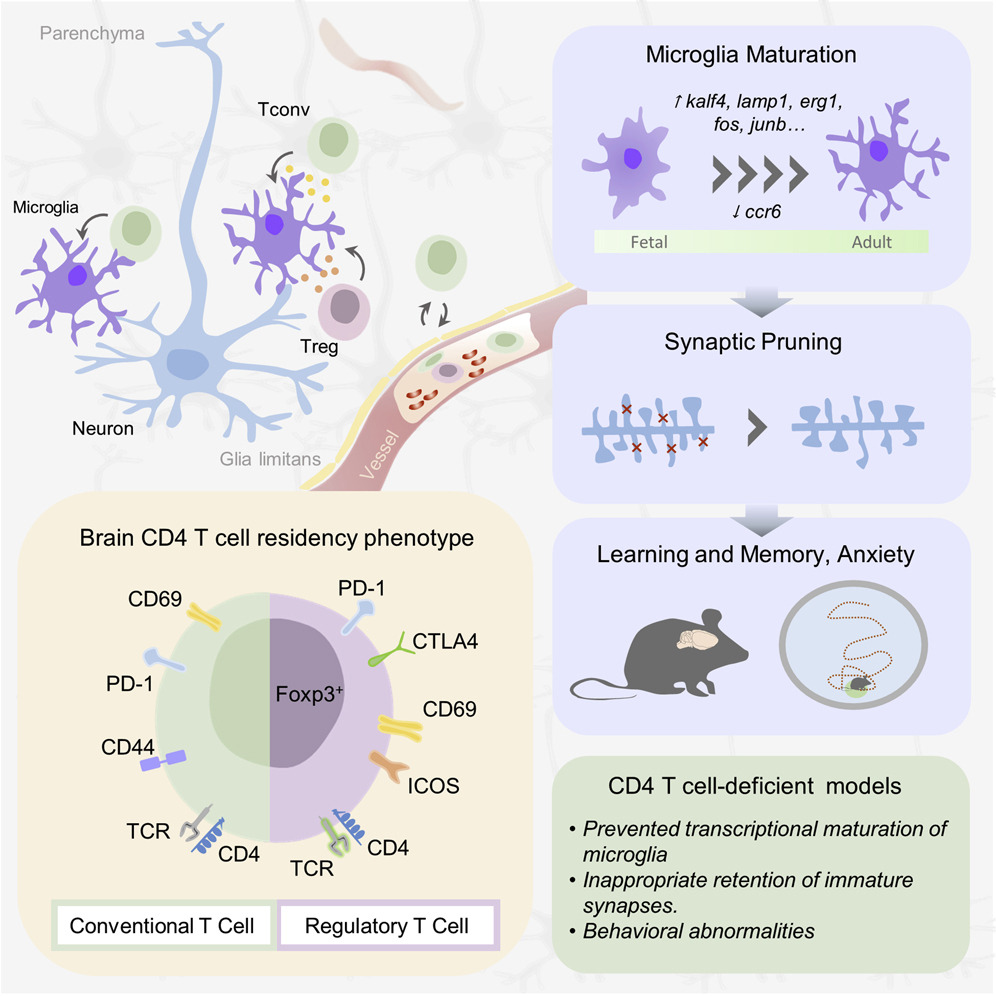 Great to see our recent Cell paper on brain T cells licensing microglia listed as one of the top 10 health innovations of 2020!
Great to see our recent Cell paper on brain T cells licensing microglia listed as one of the top 10 health innovations of 2020!
 Liston lab,
Liston lab,  immunology,
immunology,  neuroscience
neuroscience  Thursday, November 12, 2020 at 12:47PM
Thursday, November 12, 2020 at 12:47PM A team of immunology experts from Belgium and the UK research organisations have come together to apply their pioneering research methods to put individuals’ COVID-19 response under the microscope. Published today in the journal Clinical and Translational Immunology, their research adds to the developing picture of the immune system response and our understanding of the immunological features associated with the development of severe and life-threatening disease following COVID-19. This understanding is crucial to guide the development of effective healthcare and ‘early-warning’ systems to identify and treat those at risk of a severe response.

One of the most puzzling questions about the global COVID-19 pandemic is why individuals show such a diverse response. Some people don’t show any symptoms, termed ‘silent spreaders’, whereas some COVID-19 patients require intensive care support as their immune response becomes extreme. Age and underlying health conditions are known to increase the risk of a severe response but the underlying reasons for the hyperactive immune response seen in some individuals is unexplained, although likely to be due to many factors contributing together.
To investigate the immune system variations that might explain the spectrum of responses, teams of researchers from the VIB Centre for Brain and Disease Research and KU Leuven in Belgium and the Babraham Institute in the UK worked with members of the CONTAGIOUS consortium to compare the immune system response to COVID-19 in patients showing mild-moderate or severe effects, using healthy individuals as a control group.
Professor Adrian Liston, senior group leader at the Babraham Institute in the UK, explained: “One of our main motivations for undertaking this research was to understand the complexities of the immune system response occurring in COVID-19 and identify what the hallmarks of severe illness are. We believe that the open sharing of data is key to beating this challenge and so established this data set to allow others to probe and analyse the data independently.”
T he researchers specifically looked at the presence of T cells – immune cells with a diverse set of functions depending on their sub-type, with ‘cytotoxic’ T cells able to kill virus-infected cells directly, while other ‘helper’ T cell types modulate the action of other immune cells. The researchers used flow cytometry to separate out the cells of interest from the participants’ blood, based on T cell identification markers, cell activation markers and cytokine cell signalling molecules.
he researchers specifically looked at the presence of T cells – immune cells with a diverse set of functions depending on their sub-type, with ‘cytotoxic’ T cells able to kill virus-infected cells directly, while other ‘helper’ T cell types modulate the action of other immune cells. The researchers used flow cytometry to separate out the cells of interest from the participants’ blood, based on T cell identification markers, cell activation markers and cytokine cell signalling molecules.
Surprisingly, the T cell response in the blood of COVID-19 patients classified as severe showed few differences from the healthy volunteers. This is in contrast to what would usually be seen after a viral infection, such as the ‘flu. However, the researchers identified an increase in T cells producing a suppressor of cell inflammation called interleukin 10 (IL-10). IL-10 production is a hallmark of activated regulatory T cells present in tissues such as the lungs. While rare in healthy individuals, the researchers were able to detect a large increase in the number of these cells in severe COVID-19 patients.
Potentially, monitoring the level of IL-10 could provide a warning light of disease progression, but the researchers state that larger-scale studies are required to confirm these findings.
“We’ve made progress in identifying the differences between a helpful and a harmful immune response in COVID-19 patients. The way forward requires an expanded study, looking at much larger numbers of patients, and also a longitudinal study, following up patients after illness. This work is already underway, and the data will be available within months,” says Professor Stephanie Humblet-Baron, at the KU Leuven in Belgium.
 “This is part of an unprecedented push to understand the immunology of COVID-19”, concludes Professor Liston. “Our understanding of the immunology of this infection has progressed faster than for any other virus in human history – and it is making a real difference in treatment. Clinical strategies, such as switching to dexamethasone, have arisen from a better understanding of the immune pathology of the virus, and survival rates are increasing because of it”.
“This is part of an unprecedented push to understand the immunology of COVID-19”, concludes Professor Liston. “Our understanding of the immunology of this infection has progressed faster than for any other virus in human history – and it is making a real difference in treatment. Clinical strategies, such as switching to dexamethasone, have arisen from a better understanding of the immune pathology of the virus, and survival rates are increasing because of it”.
Professor Liston and Professor Humblet-Baron both emphasized the importance of the scientific team that led the study. "This work happened during a period of incredible stress. When much of our laboratory was shut down due to the pandemic, Dr Teresa Prezzemolo and Silke Janssens were in the hospital day-after-day, preparing blood samples that were critical not just for this study but for a whole host of clinical trials on COVID-19 based in Leuven. Julika Neumann and Dr Mathijs Willemsen put their PhD research on hold to run samples, and Dr Carlos Roca and Dr Oliver Burton provided the computational support to turn the data into biological understanding. We are both incredibly proud of the entire team."
Neumann, J., Prezzemolo, T., Vanderbeke, L. & Roca, C.P. et al. Increased IL-10-producing regulatory T cells are characteristic of severe cases of COVID-19. Clinical and Translational Immunology
 Coronvavirus,
Coronvavirus,  Liston lab,
Liston lab,  Medicine,
Medicine,  immunology
immunology  Wednesday, October 7, 2020 at 1:59PM
Wednesday, October 7, 2020 at 1:59PM  Emmanuelle Charpentier and Jennifer Doudna have just won the Nobel Prize for Chemistry. They have been my picks for the prize for years now. Nobel Prizes are often awarded decades after the fact, but CrispR has been such an obvious winner that it is a surprise it took until 2020 to be awarded. (Largely, I guess, due to the politics of several competing claims and patents, that have been going through the courts).
Emmanuelle Charpentier and Jennifer Doudna have just won the Nobel Prize for Chemistry. They have been my picks for the prize for years now. Nobel Prizes are often awarded decades after the fact, but CrispR has been such an obvious winner that it is a surprise it took until 2020 to be awarded. (Largely, I guess, due to the politics of several competing claims and patents, that have been going through the courts).
This Noble is a well-deserved recognition of one of the seminal breakthroughs in biology of the last several decades. The award recognises elegant basic biological experiments that identified a novel immune mechanism that bacteria use to fight off viruses. The key insight is that the chemistry of this system allowed simple modifications to rewire this bacterial system into a tool to edit the genome of essentially any living being. A striking example of blue-skies research on basic science having an incredible translational effect. The CrispR system ranks up there with identifying the structure of DNA or the sequencing of the human genome - indeed, for the first time it allows us to really use the information gained by these earlier revolutions. CrispR tools are used daily across the globe to create new vaccines, generate gene therapy, design bacteria to help industrial processes. Essentially, the discovery of CrispR as a genome-modification tool has put biology on steroids - dramatically accelerating the pace of both basic research and translational applications
 genetics,
genetics,  immunology
immunology  Wednesday, July 22, 2020 at 6:11PM
Wednesday, July 22, 2020 at 6:11PM Whether white blood cells can be found in the brain has been controversial, and what they might be doing used to be complete mystery. In a seminal study published in Cell, an international team of scientists led by Prof. Adrian Liston (VIB-KU Leuven, Belgium & Babraham Institute, UK) describe a population of specialized brain-resident immune cells discovered in the mouse and human brain, and show that the presence of white blood cells is essential for normal brain development in mice.
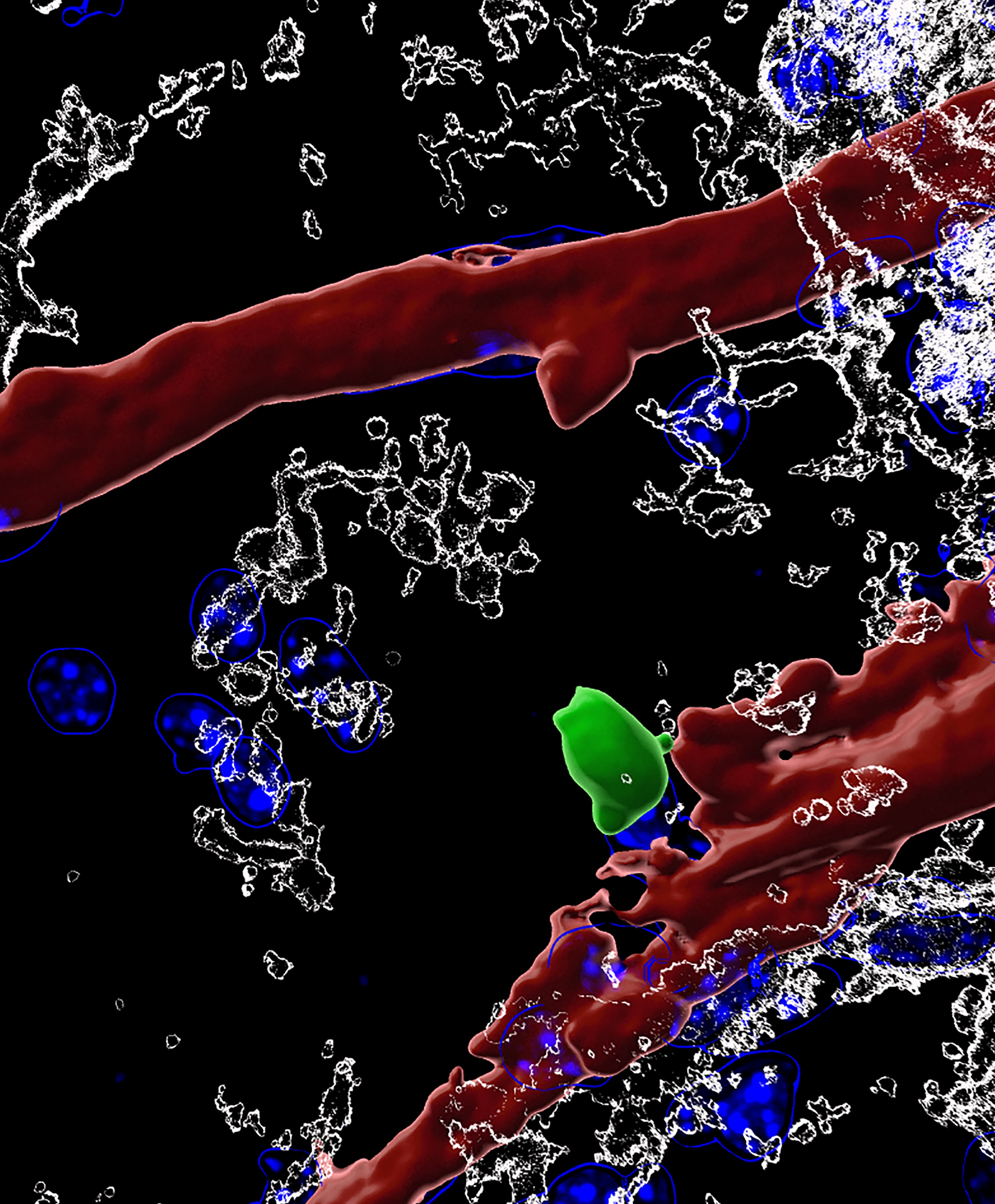 Like a highly fortified headquarters, our brain enjoys special protection from what is circulating in the rest of our body through the blood-brain barrier. This highly selective border makes sure that passage from the blood to the brain is tightly regulated.
Like a highly fortified headquarters, our brain enjoys special protection from what is circulating in the rest of our body through the blood-brain barrier. This highly selective border makes sure that passage from the blood to the brain is tightly regulated.
The blood-brain barrier also separates the brain from our body’s immune system, which is why it has its own resident immune cells, called microglia, which trigger inflammation and tissue repair. Microglia arrive in the brain during embryonic development, and later on, the population becomes self-renewing.
Yet, white blood cells—which are part of our immune system—have been found to play a role in different brain diseases, including multiple sclerosis, Alzheimer’s and Parkinson’s disease or stroke. Whether or not white blood cells can be found in healthy brains as well, and what they might be doing there, has been subject of intense debate. An interdisciplinary team of scientists led by Prof. Adrian Liston (VIB-KU Leuven, Babraham Institute) set out to find the answers.
White blood cells in the brain
"A misconception about white blood cells comes from their name,” explains Dr. Oliver Burton (Babraham Institute). “These 'immune cells' are not just present in the blood. They are constantly circulating around our body and enter all of our organs, including—as it turns out—the brain. We are only just starting to discover what white blood cells do when they leave the blood. This research indicates that they act as a go-between, transferring information from the rest of the body to the brain environment"

The team quantified and characterized a small but distinct population of brain-resident T helper cells present in mouse and human brain tissue. T cells are a specific type of white blood cells specialized for scanning cell surfaces for evidence of infection and triggering an appropriate immune response. New technologies allowed the researchers to study the cells in great detail, including the processes by which circulating T cells entered the brain and began to develop the features of brain-resident T cells.
Dr. Carlos Roca (Babraham Institute): “Science is becoming increasingly multidisciplinary. Here, we didn't just bring in expertise from immunology, neuroscience and microbiology, but also from computer science and applied mathematics. New approaches for data analysis allow us to reach a much deeper level of understanding of the biology of the white blood cells we found in the brain.”

An evolutionary role
When T helper cells are absent from the brain, the scientists found that the resident immune cells – microglia – in the mouse brain remained suspended between a fetal and adult developmental state. Observationally, mice lacking brain T cells showed multiple changes in their behavior. The analysis points to an important role for brain-resident T cells in brain development. If T cells participate in normal brain development in mice, could the same be true in humans?
“In mice, the wave of entry of immune cells at birth triggers a switch in brain development,” says Liston. “Humans have a much longer gestation than mice though, and we don't know about the timing of immune cell entry into the brain. Does this occur before birth? Is it delayed until after birth? Did a change in timing of entry contribute to the evolution of enhanced cognitive capacity in humans?”
The findings open up a whole new range of questions about how the brain and our immune system interact. "It has been really exciting to work on this project. We are learning so much about how our immune system can alter our brain, and how our brain modifies our immune system. The two are far more interconnected than we previously thought," says Dr. Emanuela Pasciuto (VIB-KU Leuven).
The study also brings in a connection with the gut microbiome, says Liston: “There are now multiple links between the bacteria in our gut and different neurological conditions, but without any convincing explanations for what connects them. We show that white blood cells are modified by gut bacteria, and then take that information with them into the brain. This could be the route by which our gut microbiome influences the brain.”
Taken together, the results contribute towards the increasing recognition of the role of immune cells in the brain and shed new light on its involvement in a range of neurological diseases.
Check out the full article here
 Liston lab,
Liston lab,  immunology,
immunology,  neuroscience
neuroscience  Wednesday, July 22, 2020 at 6:11PM
Wednesday, July 22, 2020 at 6:11PM Witte bloedcellen maken deel uit van ons immuunsysteem dat ons tegen ziektes beschermt. Of ze ook in de hersenen terug te vinden zijn, en wat ze daar dan zouden doen bleef tot nog toe een raadsel. Een internationaal team van wetenschappers onder leiding van professor Adrian Liston (VIB-KU Leuven, België en Babraham Institute, VK) toont nu aan dat witte bloedcellen wel degelijk voorkomen in de hersenen van zowel muizen als mensen, en dat hun aanwezigheid essentieel is voor normale hersenontwikkeling. De resultaten verschijnen deze week in het prestigieuze vakblad Cell.
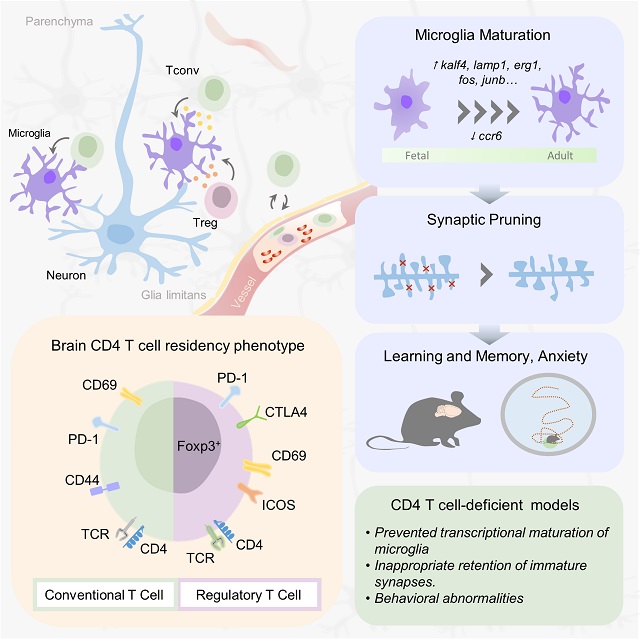 Onze hersenen worden als een versterkte burcht ommuurd door de bloed-hersenbarrière. Die moet vermijden dat stoffen die in onze bloedbaan circuleren zomaar in onze hersenen terechtkomen. Via de bloed-hersenbarrière worden onder strikte controle enkel welbepaalde stoffen uitgewisseld.
Onze hersenen worden als een versterkte burcht ommuurd door de bloed-hersenbarrière. Die moet vermijden dat stoffen die in onze bloedbaan circuleren zomaar in onze hersenen terechtkomen. Via de bloed-hersenbarrière worden onder strikte controle enkel welbepaalde stoffen uitgewisseld.
De bloed-hersenbarrière schermt de hersenen ook af van ons immuunsysteem, dat de rest van ons lichaam patrouilleert om bijvoorbeeld bacteriële of virale indringers op te sporen en uit te schakelen. Precies daarom heeft het brein z’n eigen immuuncellen: microglia.
Toch blijken witte bloedcellen ook een rol te spelen bij verschillende hersenaandoeningen. Denk maar aan MS, alzheimer, parkinson of een beroerte. Hierbij gaat het wel telkens om ziek of ‘beschadigd’ hersenweefsel, waar mogelijk ook de bloed-hersenbarrière is aangetast. De vraag bleef dus of – en waarom – witte bloedcellen nu werkelijk aanwezig zijn in hersenen die normaal en gezond zijn.
Witte bloedcellen in de hersenen
Een interdisciplinair team van wetenschappers onder leiding van prof. Adrian Liston (VIB-KU Leuven, Babraham Institute) heeft nu een kleine maar belangrijke groep van T-helpercellen ontdekt in hersenweefsel afkomstig van muizen en van mensen. T-helpercellen zijn een specifiek type witte bloedcellen, gespecialiseerd in het scannen van celoppervlakken op aanwijzingen van infectie en in het op gang trekken van een aangepaste immuunreactie. Aan de hand van de laatste technologie konden de wetenschappers de T-helpercellen tot in detail bestuderen, inclusief hoe en wanneer ze in de hersenen terecht komen.
Dr. Emanuela Pasciuto (VIB-KU Leuven), postdoctoraal onderzoeker in het team van Liston benadrukt het belang van interdisciplinair onderzoek: “Om de rol van witte bloedcellen in het brein in kaart te brengen hebben we niet alleen expertise van immunologie, neurowetenschappen en microbiologie bij elkaar gebracht, maar ook van informatica en toegepaste wiskunde. Nieuwe benaderingen voor data-analyse stellen ons in staat om een veel dieper begrip te krijgen van de biologie van de witte bloedcellen die we in de hersenen hebben gevonden.”
 Een evolutionaire rol
Een evolutionaire rol
De onderzoekers stelden vast dat in muizenhersenen zonder T-helpercellen de ontwikkeling van de typische immuuncellen van het brein (de microglia) bleef hangen ergens tussen een foetale en volwassen ontwikkelingsstatus. De muizen zonder T-helpercellen in de hersenen vertoonden bovendien verschillende gedragsafwijkingen, wat wijst op een belangrijke rol voor de T-helpercellen bij de normale hersenontwikkeling. En als dat geldt voor muizen, zou hetzelfde dan ook waar zijn voor mensen?
“We zien dat de toestroom van immuuncellen in de hersenen bij de geboorte van muizen leidt tot een omslag in het ontwikkelingsproces,” zegt Liston. “Maar de zwangerschap bij mensen is veel langer dan bij muizen, en we weten niet wanneer de immuuncellen dan toekomen in het menselijk brein. Gebeurt het nog vóór de geboorte? Is het uitgesteld tot na de geboorte? Kan een verandering in de timing bijgedragen hebben aan de evolutie van de uitzonderlijke hersencapaciteit van mensen?”
De bevindingen openen een heel nieuw gamma aan vragen over de wisselwerking tussen ons brein en ons immuunsysteem. “We leren nog elke dag bij over hoe ons immuunsysteem ons brein kan beïnvloeden en vice versa. De twee zijn veel meer met elkaar verbonden dan we eerder dachten,” zegt Pasciuto.
Darmen en hersenen
De studie legt ook nieuwe verbanden tussen ons brein en onze darmflora, aldus Liston: “Heel wat neurologische aandoeningen worden in verband gebracht met bacteriën in onze darmen, maar zonder overtuigende verklaringen voor die connectie. Onze resultaten laten zien dat darmbacteriën witte bloedcellen kunnen beïnvloeden, die deze ‘informatie’ vervolgens mee nemen naar de hersenen. Dit zou de manier kunnen zijn waarop onze darmflora onze hersenen beïnvloeden. ”
De nieuwe resultaten dragen enorm bij tot de groeiende kennis over de rol van immuuncellen in de hersenen, zowel tijdens de normale ontwikkeling als bij verschillende ziekteprocessen.
 Liston lab,
Liston lab,  immunology,
immunology,  neuroscience
neuroscience  Thursday, April 23, 2020 at 7:01PM
Thursday, April 23, 2020 at 7:01PM Severe congenital neutropenia leaves young patients to contract infection after infection, leading to life-threatening situations. A team of Leuven scientists has identified a novel genetic mutation, pointing to a new causative mechanism for this severe immune disorder.
The story starts with patient Jane Doe, now 19 years old, but diagnosed with severe congenital neutropenia when she was just 2 years old. By that time, she had already suffered an ear abscess, recurring ear infections, bronchitis, sinusitis, tonsillitis and several gum infections.
 After yet another infection, this time of her intestine, a detailed investigation revealed a striking shortage of neutrophils, white blood cells that are recruited as first-responders to the site of injury or infection within our body. Having an abnormally low concentration of neutrophils in the blood is referred to as neutropenia. When it is severe and present from birth (congenital), that is where the diagnosis of severe congenital neutropenia comes in.
After yet another infection, this time of her intestine, a detailed investigation revealed a striking shortage of neutrophils, white blood cells that are recruited as first-responders to the site of injury or infection within our body. Having an abnormally low concentration of neutrophils in the blood is referred to as neutropenia. When it is severe and present from birth (congenital), that is where the diagnosis of severe congenital neutropenia comes in.
“Severe congenital neutropenia is very scary, because these kids develop serious infections that can be lethal for infants,” explains Erika Van Nieuwenhove. “As if that’s not enough, they are also at increased risk for other conditions such as leukemia.”
Van Nieuwenhove is both an MD and PhD, who combines clinical work in the university hospital with Carine Wouters, with research at VIB and KU Leuven under the guidance of Adrian Liston and Stephanie Humblet-Baron.
Together with John Barber and several other colleagues, she set out to understand why Jane Doe developed SCN in the first place. Van Nieuwenhove: “For up to 50% of severe congenital neutropenia patients, we have no clue what causes the disease. It was the same for our patient, whose parents are both healthy.”
A new mutation in a familiar gene
After Jane Doe tested negative for mutations in all the genes with known ties to neutropenia, the researchers performed whole exome sequencing, probing every gene in the DNA, to trace back the genetic defect underlying the disorder.
“We identified a new mutation in a gene called SEC61A1, which encodes one of three subunits of the Sec61 complex. This molecular complex plays a crucial role in both protein transport and in maintaining the calcium balance of the cell,” explains Humblet-Baron. “Our experiments revealed that the genetic defect led to both a lower expression and a reduced efficacy of the SEC61A1 protein, and that these quantitative and qualitative defects in turn disturb neutrophil differentiation and maturation.”
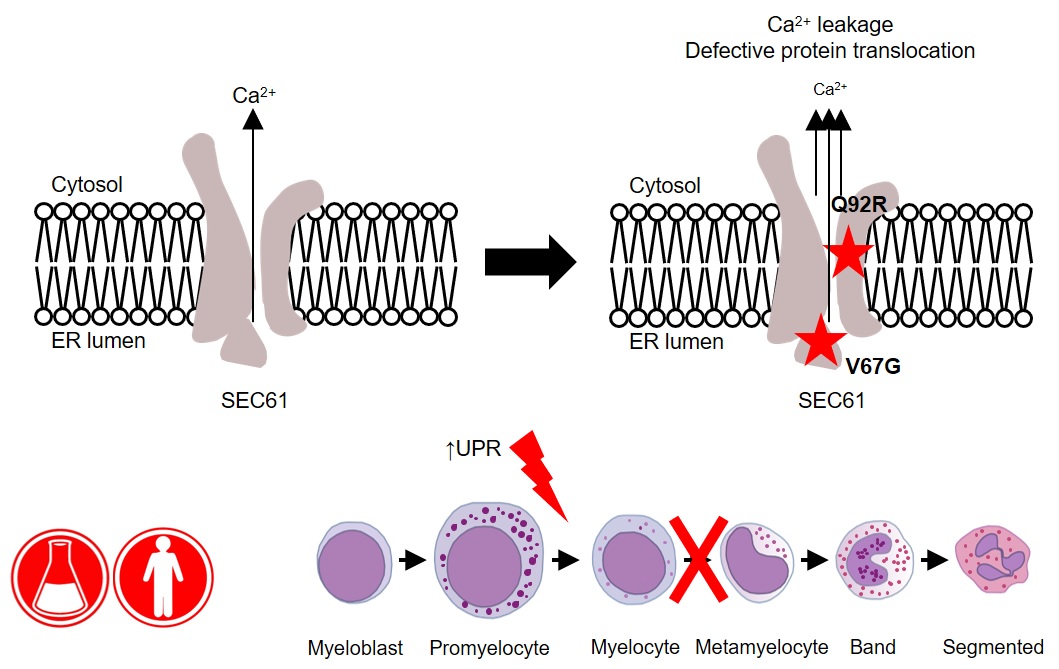
Interestingly, SEC61A1 has recently been picked up in other studies that were not focused on neutropenia. Different mutations in the same gene were reported in two families with a rare kidney disease and in two additional families with an antibody deficiency.
“The fact that there are different mutations in the same gene indicates there may be overlapping mechanisms among the different disorders. With the low number of currently known patients, it is still too early to predict which mutations can lead to which symptoms,” explains Liston.
“What’s clear from our findings is that SEC61A1 mutations can also cause severe congenital neutropenia. Considering this gene’s link with other disorders, the clinical implications of our work reach far beyond the patient with whom it all started here in Leuven.”
—
Read the original paper: Defective SEC61α1 underlies a novel cause of autosomal dominant severe congenital neutropenia. Van Nieuwenhove et al. JACI 2020
 Friday, March 27, 2020 at 12:07PM
Friday, March 27, 2020 at 12:07PM The Liston lab has collaborated with Dr Simon Andrews at Babraham Bioinformatics to create an interactive model of virus outbreak spreading. We are asking for feedback on this beta version, try it out and tweet to us at Virus Break.
To play Virus Outbreak, pick a virus (Coronavirus, flu, ebola or measles) and simulate a viral outbreak in the community. The default settings are based on real medical data, but you can modify the viral properties - change the virulence (rate of new infections), lethality, incubation period and symptomatic period. Find out why an ebola virus with the virulence of measles is the worst nightmare of virologists, run simulations of flu vs Coronavirus to see why medical experts are sounding the alarm.
In Virus Outbreak, you don't only control the virus, you control the response against the virus. Let the virus run free to create "herd immunity", or pick between vaccination, quarantine or social distancing to see what difference they can make. Change your mind on the policy? Hit "stop", go into properties to change the policy, then go back and hit "start" to see how the simulation changes. Take a look at this video where we start social distancing after the outbreak is already established:
New infections grind to a halt. It takes a week for the death rate to drop, because there are asymptomatic people built into the system, but it works! Give it a try here.